Robotic-Assisted Surgery



Manufacturers of robotic-assisted surgery devices face complex challenges that can significantly impact their efficiency, economic value, and market position. Overcoming these specific challenges requires an expert partner.

Millstone has launched four of the five leading RAS platforms, entering the market two years ahead of competitors and enabling OEM partners to reach a annual growth rate of over 30%.
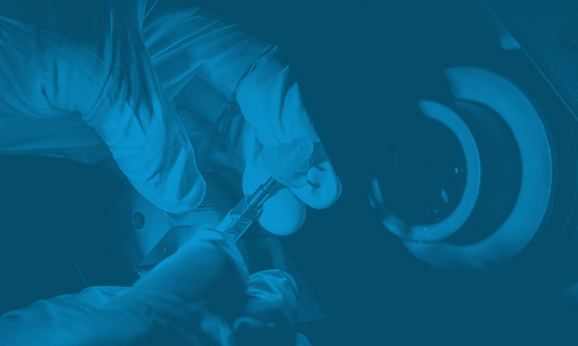
Millstone specializes in the precise assembly of complex RAS devices, ensuring seamless integration of intricate components with strict quality and regulatory compliance.
Precision Alignment of Navigation Systems
Advanced Mechanical & Electromechanical Assembly
Rigorous In-Process Quality Inspections
Supply Chain & Component Sourcing Expertise
Scalable Production for High-Volume Manufacturing
Millstone provides specialized sterile and non-sterile packaging solutions for robotic-assisted surgery (RAS) devices, ensuring compliance, precision, and speed to market in ISO-certified clean room environments.
Custom Sterile Packaging for Complex Assemblies
ISO Class 7 (10,000) Clean Room Packaging
Tray & Pouch Sealing with In-Process Integrity Testing
Vacuum & Nitrogen Flush Technology
Medical Kitting, Labeling, & Overwrapping


Millstone’s in-house testing services reduce risk, ensure compliance, and accelerate time to market by providing comprehensive validation and regulatory testing.
60% Faster Industry-Leading Turnaround
Global and FDA Regulatory Compliance
Rapid Validation In-House Testing
Sterility & Particulate Testing
Environmental & Packaging Integrity Testing
Integrated Testing – ISO 17025 Certified
Millstone provides end-to-end logistics solutions to ensure secure, efficient, and scalable distribution of RAS devices worldwide.
High-Value Inventory Management
Regulated Storage & Compliance
Real-Time Component Tracking
Just-in-Time (JIT) Distribution
Loaner Kit Management

