Why Is It Hard To Sterilize Bacterial Spores?
Sterilization: What is it?
Any procedure that eliminates, destroys, or deactivates all life is called sterilization. The word “sterile,” which denotes the total absence of live microorganisms or microbes with the capacity to proliferate, is associated with sterilization. As a result, after being put in their final packing, sterile products that go through sterilization are frequently thermally or chemically sterilized. Any bacteria found within the products (obtained during manufacture and packaging) are eliminated by chemical or heat sterilization. Terminal sterilization is the process of sterilizing a product using heat or chemicals after it has been packaged.
There are four primary ways to sterilize objects:
- Heat
- Gas
- Radiation
- Filtration
These four sterilizing techniques can also be categorized as follows:
- Thermal
- Moist
- Dry
- Nonthermal
- Filtration
- Radiation
- Chemical
- Gaseous
- Liquid
Which microbes need to be eradicated by sterilizing procedures?
Three main types of microorganisms are eliminated by sterilization procedures: viruses, fungi (such as mold and yeast), and bacteria.
What are bacterial spores?
Only certain gram-positive bacterial species produce bacterial spores. The absence of an outer cell wall distinguishes gram-positive bacteria from gram-negative bacteria. Common gram-positive bacteria include Clostridium, Bacillus, Streptococcus, and Staphylococcus. When stressed bacterial cells create an outer shell to shield themselves from harmful environmental factors like heat, pollutants, and food deficiency, bacterial spore formation takes place. Bacillus and Clostridium species are the most prevalent spore formers. Keep in mind that gram-positive bacteria are the common spore formers. The problem with bacterial spore forms is that they are hundreds of times harder to eradicate than regular bacteria. Therefore, it is essential to confirm sterilizing procedures employing spore-forming bacteria (or their equivalents) as biological markers. In fact, a sterilizing procedure will be able to eradicate other resident bacteria if it is successful in killing specific concentrations of spore-forming bacteria.
Why is it difficult to eradicate bacterial spores?
The production of bacterial spores is a coping strategy used by cells in hostile environments. Bacterial cells use spore formation as a defense mechanism to preserve their energy. Bacterial spores are a dormant form of bacteria because they are in a condition of hibernation, which conserves energy. They produce fewer enzymes, have a low respiration rate, and a limited metabolism. The most well-known function of gram-positive bacteria is spore formation. Within a bacterial cell that forms spores, spores can form in several different places. We refer to these internal spores as endospores.
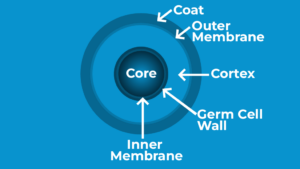
Figure 1: Endospore Diagram
The endospore’s cortex and core are encased in a thick-walled proteinaceous outer shell, as shown in Figure 1. The majority of the endospore’s defense against chemicals and enzymes is provided by its proteinaceous covering. A thick layer of peptidoglycan covers the cortex beneath the endospore’s covering. The cortex enables the endospore to withstand high temperatures and dehydrates the spore core. After the endospore germinates, the germ cell wall located beneath the cortex will develop into the bacterium’s cell wall. The endospore’s core contains the ribosomes and DNA of the cell. Additionally, the center has tiny, acid-soluble proteins that help the endospore withstand damage from UV rays and substances that damage DNA.
It takes several hours for endospores to form. The bacterial cell divides asymmetrically in the first stage, producing a mother cell and a forespore. The mother cell then absorbs the forespore, creating a cell inside a cell. The cortex then develops around the core of the forespore, and the proteinaceous coat then forms around the cortex. Following the endospore’s maturity and dehydration, the mother cell starts the process of programmed cell death. After then, the endospore stays dormant until the right environmental circumstances are present.
As previously stated, endospores can withstand temperatures as low as almost absolute zero and as high as 150°C, making them quite challenging to eradicate. Endospores can withstand high pH gradients, drought, UV light, chemical agents (including alcohol), and a lack of nutrients. Endospores emerge from their dormant state and re-emerge as active bacterial cells with regular metabolisms when the environmental conditions are favorable for bacterial growth. Bacterial spores can be eliminated in two ways. The first is that in order to kill the bacterial spore cells using conventional sterilizing methods, the environmental circumstances must induce them to return to their active bacterial state. The availability of nutrients, the transport of water through bacterial cell walls, and temperatures near 37°C are all factors that cause spore germination to return to an active state. As an alternative, spores can be eliminated without triggering spore germination by using harsh sterilization techniques or extremely high temperatures.

In brief
In general, sterilization refers to any procedure that eliminates, destroys, or deactivates all living things. In order to guarantee patient safety when using medical devices and products, sterilization is essential. Before using medical devices, three main types of microbes must be properly sterilized (elimination): viruses, fungi (yeast and mold), and bacteria. Spore forms of bacteria are the most challenging to eradicate among these three germ kinds. Bacterial endospores may withstand temperatures as low as almost absolute zero and as high as 150°C, making spore forms of bacteria difficult to eradicate. Endospores are also resistant to high pH gradients, drought, UV light, chemical agents (including alcohol), and nutritional depletion. Nonetheless, there are two ways to eliminate bacterial endospores. The first technique involves destroying them in their spore condition at high temperatures. The second technique involves stimulating endospore germination in order to kill active bacterial cells, which are readily eliminated by conventional sterilizing techniques. Overall, make sure the contract testing company you select can offer the right sterilizing validations for your product requirements.






